Device by PUHS Research Group Received Approval for Marketing
A customized 1-piece glass fiber post and core, fabricated using a CAD/CAM system, received approval from the National Medical Products Administration (NMPA) for marketing as Class III Medical Device (No. 20213170796). The device was a translation from the original study conducted ten years ago by Prof. Deng Xuliang’s group at the Peking University Hospital of Stomatology (PUHS), and was developed by the PUHS, Beijing University of Chemical Technology, and Beijing OYA Ricom New Material Sci.&Tech. Co., Ltd.
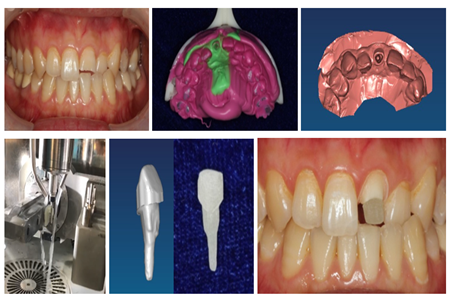
As a product of independent innovation, this new device brought about an alternative treatment strategy for fractured teeth. It was also a success of collaboration among hospitals, research institutions and the industry that brought research results from bench to bedside.
Written by: Fan Xiaofei
Edited by: Liu Xin


